Glossary of Symbols Used
Symbols used int the product labeling of Smooth-Bor Plastics medical devices
| Symbol | Identification | Description |
|---|---|---|
 |
Manufacturer | Indicates the device manufacturer per EU Directives 93/42/EEC, 90/385/EEC and 98/79/EC. |
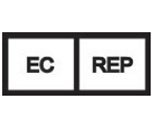 |
European Union Representative | The authorized representative for Smooth-Bor Plastics in the European Union. |
 |
Catalogue Part Number | Smooth-Bor Plastics’ catalogue Number |
 |
Lot Number | Traceable control number for the batch of products. |
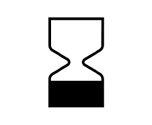 |
Expiry Date | Indicates the date after the medical device is not to be used. |
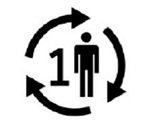 |
Single Patient Use | Part is not to be used by multiple patients. |
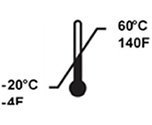 |
Temperature Limits | Indicates the upper and lower temperature limits for the handling, storage of the device |
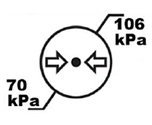 |
Atmospheric Pressure Limits | Indicates the upper ana lower atmospheric pressure limits with the device can be safely exposed to. |
 |
Consult Instruction for Use | See instructions for use. |
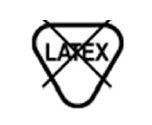 |
Latex Free | Not made with natural rubber latex. |
 |
See User’s Manual | See user manual and/or operators manual. |
 |
Prescription Only | USA Regulatory mark for “Federal law restricts this device to sale by or on the order of a physician.” |

